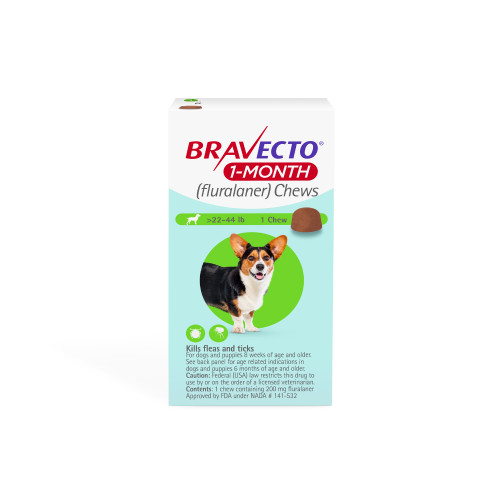
Product details
Vet-recommended and FDA-approved BRAVECTO 1-MONTH (fluralaner) Chews keeps your puppy protected from flea infestations, black-legged ticks, American dog ticks, brown dog ticks, Asian longhorned ticks, and lone star ticks*. Designed for puppies at least 8 weeks old and weighing 4.4 pounds or more, this tasty, pork-flavored chew is easy to administer and convenient. With BRAVECTO 1-MONTH Chews, you can trust that your puppy will be well protected and prepared—no matter what life has in store for your puppy.
-
Safe and easy treatment
-
Designed for puppies at least 8 weeks old, weighing 4.4 pounds or more
-
One month of protection against fleas and ticks
-
Tasty, pork-flavored chew
-
Available in four sizes based on your puppy's weight
*BRAVECTO 1-MONTH Chews kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of tick infestations (Ixodes scapularis [black-legged tick], Dermacentor variabilis [American dog tick], Rhipicephalus sanguineus [brown dog tick], and Haemaphysalis longicornis [Asian longhorned tick]) for one month in dogs and puppies 8 weeks of age and older and weighing 4.4 pounds or greater.
BRAVECTO 1-MONTH Chews are also indicated for the treatment and control of Amblyomma americanum (lone star tick) infestations for one month in dogs and puppies 6 months of age and older and weighing 4.4 pounds or greater.
Product sourced directly from the manufacturer or their approved distributor. Guaranteed genuine product backed by the manufacturer.
For
Dogs and Puppies (8 weeks of age and older)
Active ingredient(s)
Fluralaner
Inactive ingredients
Complete inactive ingredients list not provided by manufacturer.
Brand name
BRAVECTO 1-MONTH Chews
Drug class
Isoxazoline ectoparasiticide
Product flavoring
BRAVECTO 1-MONTH Chews are pork flavored.
*Contains pork, corn, soy
Manufacturer
Merck Animal Health
Product strength & size
|
Weight Range
|
Fluralaner content
|
Chews administered
|
|---|
|
4.4 to 9.9 lbs
|
45 mg
|
One
|
|
>9.9 to 22 lbs
|
100 mg
|
One
|
|
>22 to 44 lbs
|
200 mg
|
One
|
|
>44 to 88 lbs
|
400 mg
|
One
|
For dogs over 88 lbs, administer the appropriate combination of chews to cover your dog’s body weight.
Usage
*For complete product information, please review the full prescribing information in the manufacturer package insert.
BRAVECTO 1-MONTH Chews should be given orally as directed by your veterinarian. Ensure the entire dose is consumed, and no part of the dose is lost or refused.
Manufacturer Dosage and Administration:
Bravecto 1-Month should be administered orally as a single monthly dose according to your dog’s body weight to provide a minimum dose of 4.5 mg/lb (10 mg/kg) fluralaner.
Bravecto 1-Month should be administered with food.
Treatment with Bravecto 1-Month may begin at any time of the year and can continue year-round without interruption.
Missed doses
If a dose is missed: give BRAVECTO 1-MONTH as soon as you remember, then wait for the full prescribed dosing interval prior to giving the next dose. Do not give two doses at once.
If you have questions about your pet’s prescribed dosing schedule (e.g., timing, interval), please consult your veterinarian.
Storage
Store in a cool, dry place at a controlled room temperature (at or below 86°F). Keep out of the reach of children and pets. Tablets should remain sealed in the original packaging to protect from light and only taken out of the blister pack at the time of dosing. Store away from heat and direct sunlight.
Do not store in the bathroom, near the kitchen sink, or in damp places. The medicine may break down if exposed to heat or moisture.
Side effects
BRAVECTO 1-MONTH is generally well tolerated. The most common side effects of this medication are vomiting, diarrhea, decreased appetite, drowsiness/lethargy, and itching.
Contact your veterinarian immediately if your pet experiences severe or persistent vomiting, complete loss of appetite, has a seizure or any other neurologic adverse reaction (e.g., tremors, loss of coordination, weakness), or if any signs of a hypersensitivity reaction (e.g., hives, swelling) occur.
Notify your veterinarian if your pet experiences any of the effects described above, or if you notice any other side effects that are persistent or troublesome.
If you notice anything unusual, please consult your veterinarian.
Precautions
*For complete product information including warnings and precautions, please refer to the manufacturer package insert.
For oral use in dogs only- not for human use. Keep this and all drugs out of the reach of children. Keep the product in the original packaging until use to prevent children from getting direct access to the product. Do not eat, drink or smoke while handling the product. Wash hands thoroughly with soap and water immediately after use of the product.
Keep Bravecto 1-Month in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Fluralaner is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders.
Bravecto 1-Month is not effective against A. americanum (lone star ticks) in puppies less than 6 months of age.
The safety of Bravecto 1-Month has not been evaluated in breeding, pregnant, and lactating dogs. Discuss the risks of using this medication with your veterinarian prior to administration if your pet is in one of these groups.
Do not administer to animals with a known history or suspected allergy/hypersensitivity to any isoxazoline class drug, including this medication or any of its ingredients. Allergic reactions to medications may occur. Be sure to inform Vetsource and your veterinarian if your pet has any known drug sensitivities or allergies.
If your pet displays symptoms of an allergic reaction, discontinue therapy and call your veterinarian immediately or seek emergency veterinary attention. Symptoms may include (but are not limited to): swollen lips, tongue, face, or airways; difficulty breathing; agitation; profuse salivation; and widespread hives or itching.
Drug and food interactions
There are no documented drug or food interactions with this medication.
Contact your veterinarian if your pet experiences any unusual reactions when different medications are given together.
Please ensure your veterinarian is aware of all medications and supplements that your pet is currently receiving. Your veterinarian may prescribe multiple medications, even if a potential drug interaction may occur. In these instances, your veterinarian may adjust the dosages or monitor your pet more closely.
Adverse reactions
If you are concerned that your pet has experienced an adverse reaction to this medication, please contact the manufacturer Merck Animal Health at 1-800-224-5318 or Vetsource Pet Owner Care at 877-738-4443.
Overdose
If you have any reason to suspect an overdose, call your doctor/veterinarian or the appropriate poison control resource immediately.
For humans:
The national toll-free Poison Help line, 1-800-222-1222, will connect you to your local poison center in case of emergency. This service is available nationwide and in most U.S. territories.
For animals:
The ASPCA Animal Poison Control Center is available 24 hours a day, 365 days a year at 888-426-4435.
Pet Poison Helpline® also provides a 24/7 animal poison control service at 855-764-7661.
*Please note: this information is for third-party services and is provided for convenience in case of potential poison-related emergencies. There may be consultation fee for these services.
Disclaimer
The content provided on this page is NOT medical advice.
All content, including the images and product description above, is intended for general informational purposes only and should not be considered a substitute for professional veterinary consultation, diagnosis, or treatment.
Consult your veterinarian for complete information about this product and how it fits into your pet's individual treatment plan.
Last revised: 8/12/2025